Unveiling the Yeast Two-Hybrid Assay: A Powerful Tool for Protein-Protein Interaction Studies
The yeast two hybrid assay is a versatile method primarily designed to study the interactions between two proteins. It is based on the principle of transcriptional activation in yeast cells, where two fusion proteins are expressed: one containing a DNA-binding domain and the other a transcriptional activation domain. When the two proteins interact, they bring together these two domains, leading to the activation of reporter genes. The presence of a reporter gene allows for the easy detection and quantification of the interaction.
One of the most significant advantages of the yeast two-hybrid assay is its ability to detect protein interactions in a eukaryotic environment. Yeast cells provide a suitable system that mimics the cellular machinery of higher organisms, making it easier to study complex protein interactions that may be overlooked in prokaryotic systems.
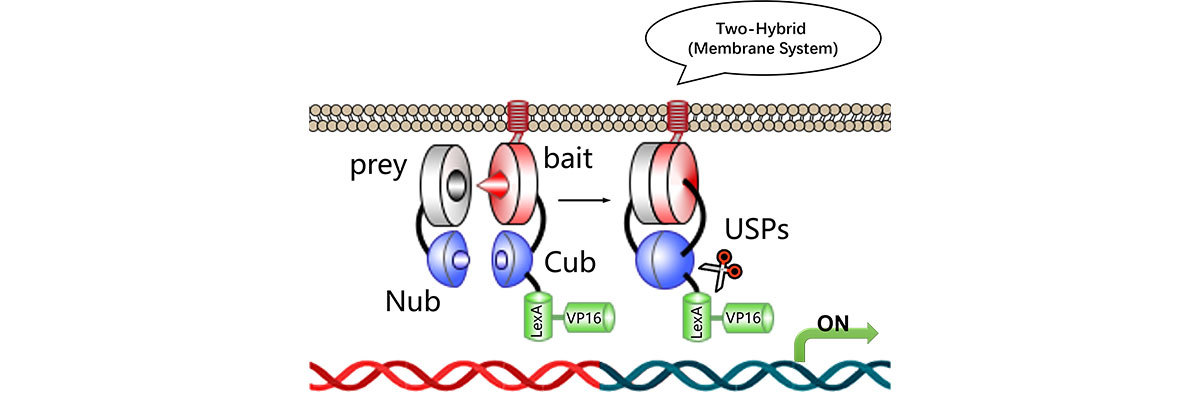
In addition to identifying direct interactions, the yeast two-hybrid assay can also be employed to map interaction domains and characterize the specificity of these interactions. Researchers can create libraries of proteins or protein fragments, which can then be screened against a target protein of interest. This high-throughput capability significantly accelerates the discovery of novel protein interactions.
The yeast two-hybrid assay has also proven invaluable in drug discovery and development. By identifying key protein interactions that contribute to disease processes, researchers can target these interactions with small molecules or biologics, leading to the development of more effective therapeutic strategies.
Furthermore, understanding the interactome of a particular disease can provide insights into the underlying biological pathways involved, facilitating the discovery of new biomarkers for diagnostics.

While the yeast two-hybrid assay is a powerful tool, it is essential to recognize its limitations. The assay may produce false positives or negatives due to various factors, such as the expression levels of the proteins involved, the suitability of the yeast strain used, or even the conditions under which the assay is performed.
Therefore, it is crucial to validate the interactions observed through complementary methods, such as co-immunoprecipitation or fluorescence resonance energy transfer (FRET).